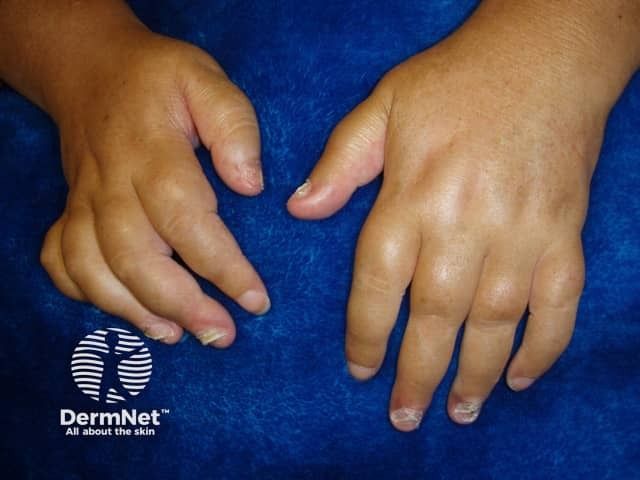
Protagonist Announces Submission of NDA for First Icotrokinra U.S. FDA Approval Aiming to Revolutionize Treatment ...
Icotrokinra is a first-in-class investigational targeted oral peptide that selectively blocks the IL-23 receptor Filing based on unprecedented data package that met all primary endpoints across four Phase 3 studies, including head-to-head superiority …